New Research Sheds Light on Stem Cell Repair Mechanisms
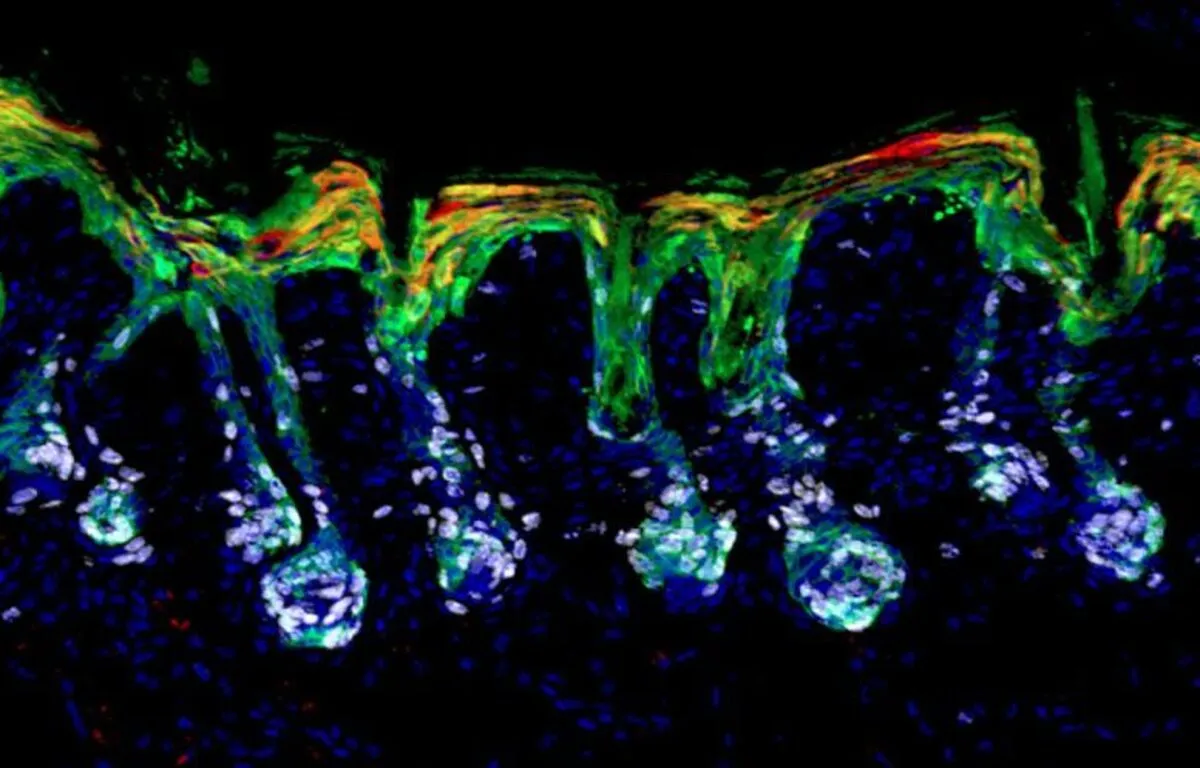
In a screen to identify key regulators of this process, retinoic acid, the biologically active form of Vitamin A, surfaced as a surprising rheostat. The findings shed light on lineage plasticity, with potential clinical implications, the journal Science reported.
“Our goal was to understand this state well enough to learn how to dial it up or down,” says Rockefeller’s Elaine Fuchs. “We now have a better understanding of skin and hair disorders, as well as a path toward preventing lineage plasticity from contributing to tumor growth.”
Lineage plasticity has been observed in multiple tissues as a natural response to wounding and an unnatural feature of cancer. But minor skin injuries are the best place to study the phenomenon, because the skin’s outer layers are subject to perpetual abuse. And when the scratches or abrasions damage the epidermis, hair follicle stem cells are the first responders.
Fuchs and colleagues began to look more closely at lineage plasticity because it, “can act as a double-edged sword,” explains Matthew Tierney, lead author on the paper and an NIH K99 “pathway to independence” postdoctoral awardee in the Fuchs lab. “The process is necessary to redirect stem cells to parts of the tissue most in need but, if left unchecked, it can leave those same tissues vulnerable to chronic states of repair and even some types of cancer.”
To better understand how the body regulates this process, Fuchs and her team screened small molecules for their ability to resolve lineage plasticity in cultured mouse hair follicle stem cells, under conditions that mimicked a wound state. They were surprised to find that retinoic acid, a biologically active form of vitamin A, was essential for these stem cells to exit lineage plasticity and then be coaxed to differentiate into hair cells or epidermal cells in vitro.
“Through our studies, first in vitro and then in vivo, we discovered a previously unknown function for vitamin A, a molecule that has long been known to have potent but often puzzling effects on skin and many other organs,” Fuchs says. The team found that genetic, dietary, and topical interventions that boosted or removed retinoic acid from mice all confirmed its role in balancing how stem cells respond to skin injuries and hair regrowth. Interestingly, retinoids did not operate on their own: their interplay with signaling molecules such as BMP and WNT influenced whether the stem cells should maintain quiescence or actively engage in regrowing hair.
The nuance did not stop there. Fuchs and colleagues also demonstrated that retinoic acid levels must fall for hair follicle stem cells to participate in wound repair—if levels are too high, they fail to enter lineage plasticity and can’t repair wounds—but if the levels are too low, the stem cells focus too heavily on wound repair, to the expense of hair regeneration.
“This may be why vitamin A’s effects on tissue biology have been so elusive,” Fuchs says.
One result of retinol biology remaining obscure for so long is that retinoid and vitamin A applications have long produced confusing results. Topical retinoids are known to stimulate hair growth in wounds, but excessive retinoids have been shown to prevent hair cycling and cause alopecia; both positive and negative effects of retinoids on epidermal repair have been documented through various studies. The present study brings greater clarity by casting retinoids in a more central role—at the helm of regulating both hair follicle and epidermal stem cells.
“By defining the minimal requirements needed to form mature hair cell types from stem cells outside the body, this work has the potential to transform the way we approach the study of hair biology,” Tierney says.
How retinoids impact other tissues remains to be seen. “When you eat a carrot, vitamin A gets stored in the liver as retinol where it is sent to various tissues,” Fuchs says. “Many tissues that receive retinol and convert it to retinoic acid need wound repair and use lineage plasticity, so it will be interesting to see how broad the implications of our findings in skin will be.”
The Fuchs lab is also interested in how retinoids impact lineage plasticity in cancer, particularly squamous and basal cell carcinoma. “Cancer stem cells never make the right choice—they are always doing something off-beat,” Fuchs says. “As we were studying this state in many types of stem cells, we began to realize that, when lineage plasticity goes unchecked, it’s a key contributor to cancer.”
Basal cell carcinomas have relatively little lineage plasticity and are far less aggressive than squamous cell carcinomas. If future studies demonstrate that suppressing lineage plasticity is key to controlling tumor growth and improving outcomes, retinoids may have a key role to play in treating these cancers.
“It’s possible that suppressing lineage plasticity can improve prognoses,” Fuchs says. “This hasn’t been on the radar until now. It’s an exciting front to now investigate.”
4155/v





















