Photosynthesis of Green Methanol from Methane
Green methanol is regarded as a future sustainable feedstock for the hydrogen economy as a liquid hydrogen carrier, and a precursor for around 30% of known commodity chemicals. Currently, it is manufactured from fossil-sourced syngas, a stoichiometrically defined mixture of carbon monoxide (CO) and hydrogen (H2), the journal Nature Materials reported.
A holy grail in this field is to make methanol from the methane component of natural gas, however, the exceptionally high carbon-hydrogen (C-H) bond dissociation energy of methane (CH4, 439 kJ/mol) and ease of partial and complete oxidation of methanol (CH3OH) to carbon monoxide (CO) and carbon dioxide (CO2), presents a grand challenge in the search for catalysts that can selectively convert CH4 to CH3OH.
Yet, in nature this feat is enabled under ambient conditions by the di-iron catalytic hydroxylation sites in the enzyme called methane monooxygenase, which is found in methanotrophic bacteria.
Many attempts have been made to mimic the efficacy of the methane monooxygenase biocatalyst in the laboratory, but no artificial version has, until very recently, matched the conversion yield, selectivity, and long-term performance metrics of nature’s CH4 to CH3OH system.
Somewhat counterintuitively, it has been discovered that a mono-iron site chemically integrated into the pore walls of a metal organic framework (MOF) in the presence of light under ambient conditions in a stream of water (H2O) and oxygen (O2) can catalytically hydroxylate CH4 to CH3OH with 100% selectivity at a conversion rate of 8.81 mmol/gcat.hr. This represents a 75% performance improvement in the conversion rate compared to nature’s methane monooxygenase.
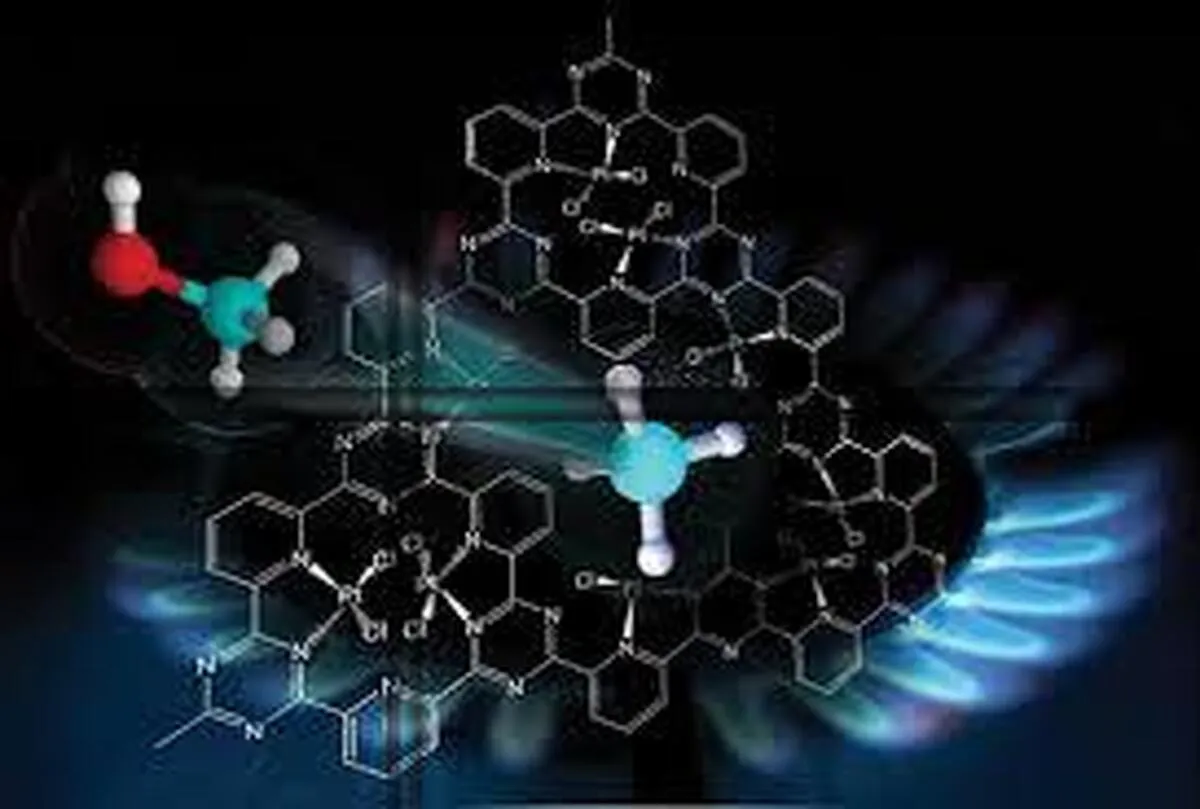
To amplify, a scheme of the molecule and materials chemistry design, synthesis, structural and operational principles of this impressive methane hydroxylation photocatalyst is encapsulated in the figure. The MOF selected to host the key mono-iron catalytic hydroxylation site is dubbed UiO-67, which is a large pore zirconium oxo-cluster based open framework material.
Three judiciously selected functional components have been assembled through creative chemical synthesis into the UiO-67 platform to provide the system with the ability to absorb light, provide multi-electron redox activity, and a mono-iron hydroxide site to activate CH4.
4155/v





















