Iranian Knowledge-Based Firm Settles Challenge of Treating Diabetic, Chronic Wounds
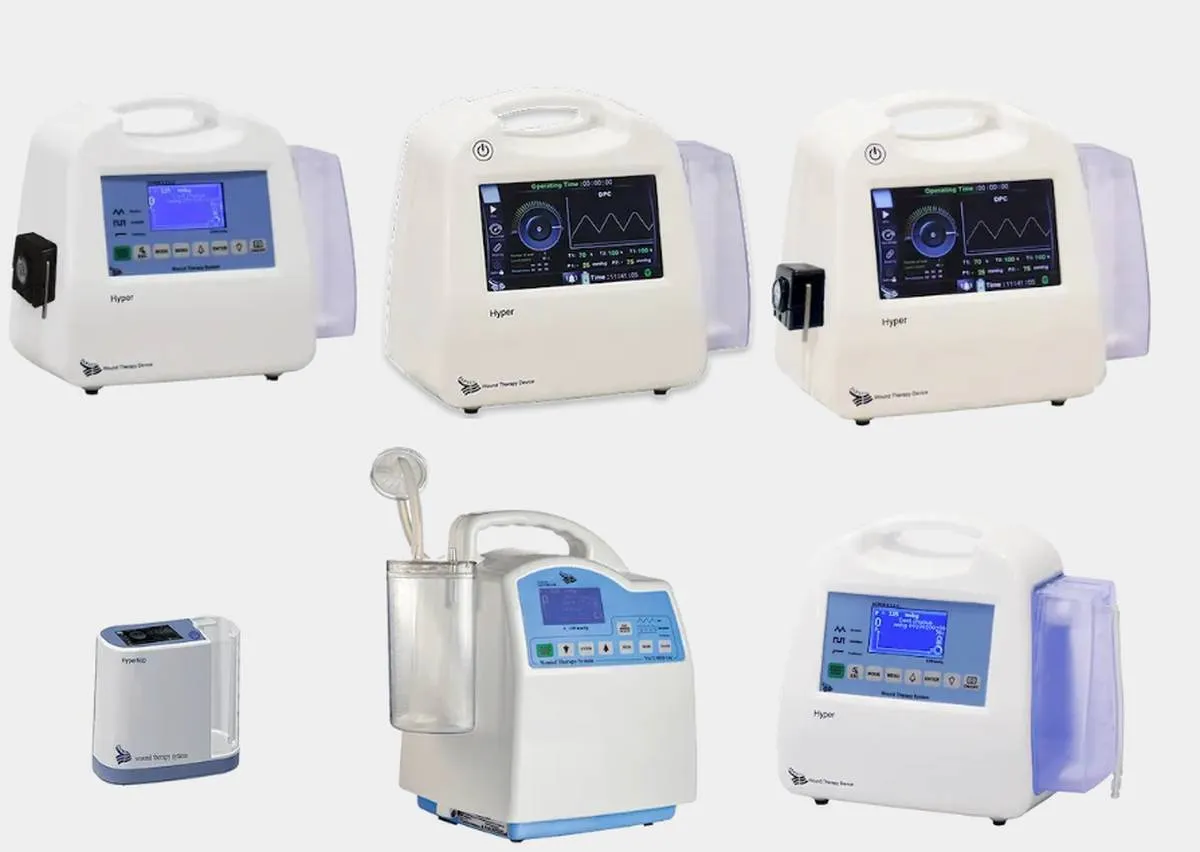
“Using the latest technology and the knowledge of our experts, our company has succeeded in receiving the European Standard (CE) certificate for the production of vacuum wound therapy, suction, and consumables,” said Seyed Ahmad Hosseini, the managing director of the knowledge-based company.
“The product for which we received the Knowledge-Based Technology Award is a vacuum wound therapy device and its consumables. At present, over seven million people in Iran have diabetes, and there are thousands of amputations due to diabetic wounds every year because these wounds cannot be treated with conventional methods,” he added.
“Vacuum therapy is one of the most effective treatments for chronic, pressure, diabetic, and other grade 2, 3, and 4 wounds. This method has been used for the treatment of chronic wounds for about 40 years around the world,” Hosseini said.
“The vacuum therapy device manufactured by our company is equipped with a remote control application and the treatment process can be monitored remotely,” he noted.
In a relevant development in September, Iranian researchers at a knowledge-based company, affiliated to Royan Research Institute, presented two skin products to the pharmacies across the country which are used to treat diabetic wounds and the 3rd degree burns.
“This knowledge-based company is active in production of multiple cell, tissue and gene therapy products. Recently, two skin products which are tissue cell sheets have entered the country's official pharmaceutical list,” Sara Pousti, a researcher of the knowledge-based company, told ANA.
“These products are used for people who have severe diabetes and their diabetic foot ulcers lead to amputation, or people who have hard jobs and have suffered third degree burns and deep bruises,” she added.
“When these cell sheets are transplanted on the burn area or diabetic foot wounds, they cause complete restoration of the lost tissue and prevent amputation,” Pousti noted, adding that the safety, sterility, identity, effectiveness, degree of purity and performance of the final product of these two products have been approved by experts through various quality control tests.
4155/v





















